Our research covers current prescribing practice, policy initiatives, interventions across hospitals and primary healthcare, and much more.
Prof Mike Sharland chaired the antibiotic working group on the WHO Essential Medicines Committee responsible for developing the AWaRe classification in 2017 and the subsequent development of the wider AWaRe system, including the WHO 2022 AWaRe Antibiotic Book.
In 2024, the UN General Assembly on AMR agreed that 70% of global antibiotic use should be Access antibiotics.
The aim of the Antibiotic Policy Group is to assist policymakers, clinicians and all relevant stakeholders with analyses and tools that can be used to inform antibiotic policies and interventions to improve antibiotic use.
Read the full list of AWaRe antibiotics
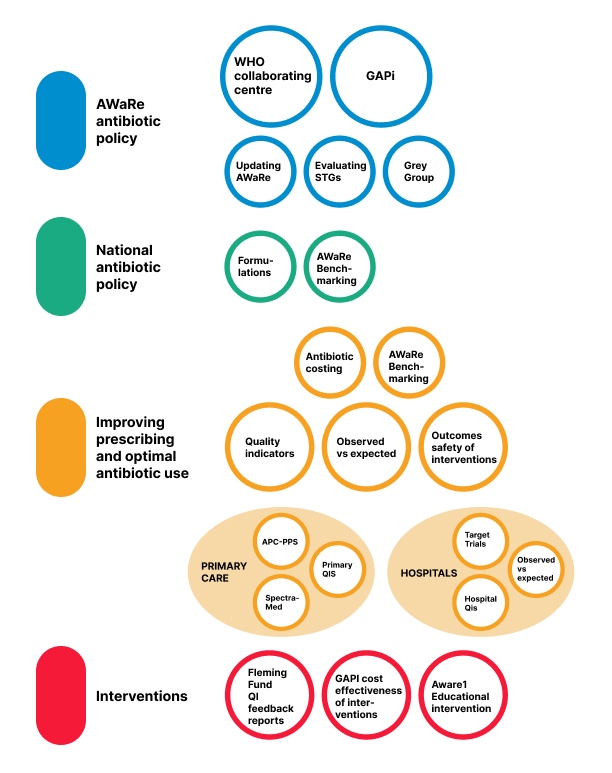
Browse our projects or use the filter

AWaRe 1 Trial
Primary Care Antimicrobial Stewardship Trial in Africa and Asia using the WHO AWaRe System
Explore our project outputs
ADILA: Target trial- Cordant Treatment
Evaluating the impact of delayed concordant antibiotic treatment on 30-day mortality in patients with bloodstream infection in LMICs

ADILA: Advancing monitoring of antimicrobial resistance trajectories using flexible spatiotemporal modelling
A change-point analysis

ADILA: Spectramed
Antibiotic prescribing for common acute infections in low-middle income countries – an analysis using IQVIA prescriber surveys from Pakistan, Egypt, and Indonesia
ADILA: Quality Indicators
Development of AWaRe (Access, Watch, Reserve) antibiotic quality indicators for optimal use

ADILA: Global pricing of AWaRe antibiotics
Global pricing of AWaRe (Access, Watch, Reserve) antibiotics: implications of the UNGA-AMR 70% Access target on national pharmaceutical expenditure

ADILA: Formulations
Using oral and parenteral formulation of AWaRe antibiotics as a proxy estimate of primary healthcare and inpatient hospital sector use

ADILA: Benchmarking AWaRe
Estimating optimal AWaRe antibiotic use for 186 countries based on infection and resistance burden

ADILA: No antibiotic care (CPRD study)
Association between propensity to prescribe antibiotics for common infections in primary healthcare and adverse outcomes

ADILA: CAP Systematic Review
Systematic review on clinical efficacy of amoxicillin for treatment of community acquired pneumonia

ADILA: Observed and expected antibiotic use
Comparing Observed versus Expected Empiric Antibiotic Use in Hospitals
Lorem ipsum
Lorem ipsum